Page 8 - Vacchi_Marriner_2016
P. 8
M. Vacchi et al. / Earth-Science Reviews 155 (2016) 172–197 179
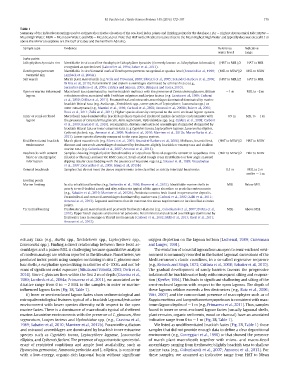
Table 1
Summary of the indicative meanings used to estimate the relative elevation of the sea-level index points and limiting points for the database. HAT — Highest Astronomical Tide; MHW —
Mean High Water; MLW — Mean Low Water; and MSL — Mean Sea Level. Note that HAT in the Mediterranean is close to the Mean Highest High Water and typically does not exceed 0.1 m
above the MHW (exceptions are the Gulf of Gabes and the Northern Adriatic).
Sample type Evidence Reference Indicative
water level range
Index points
Lithophyllum byssoides rim Identifiable in situ coralline rhodophyte Lithophyllum byssoides (formerly known as Lithophyllum lichenoides) (HAT to MSL)/2 HAT to MSL
recognized at species level (Laborel et al., 1994; Faivre et al., 2013).
Dendropoma petraeum Identifiable in situ Vermetid reefs of Dendropoma petraeus recognized at species level (Antonioli et al., 1999; (MSL to MLW)/2 MSL to MLW
Vermetid rim Lambeck et al., 2004a).
Salt marsh Marsh plant macrofossils (e.g. Vella and Provansal, 2000; Silvestri et al., 2005; Serandrei-Barbero et al., 2006; (HAT to MSL)/2 HAT to MSL
Di Rita et al., 2010). Foraminiferal and diatom assemblages dominated by saltmarsh taxa (e.g.,
Serandrei-Barbero et al., 2006; Caldara and Simone, 2005; Blázquez and Usera, 2010).
Open or marine influenced Macrofossil taxa dominated by marine brackish molluscs with the presence of Cerastoderma glaucum, Bittium −1 m MSL to –2m
lagoon reticulatum often associated with Cerithium vulgatum and Loripes lacteus (e.g. Gravina et al., 1989; Carboni
et al., 2010; Di Rita et al., 2011). Foraminiferal and ostracods assemblages dominated dominated by marine
brackish littoral taxa (e.g. Aurila spp., Xestoleberis spp., some species of Leptocythere; Loxoconcha spp.) or
outer estuary taxa (e.g., Mazzini et al., 1999; Carboni et al., 2002; Amorosi et al., 2008b; Ruiz et al., 2006;
Rossi et al., 2011; Zaîbi et al., 2011). Higher species diversity compared to the semi-enclosed lagoon system.
Inner or semi-enclosed Macrofossil taxa dominated by brackish molluscs typical of sheltered marine-lacustrine environments with −0.5 m MSL to −1m
lagoon the presence of Cerastoderma glaucum, Abra segmentum, Hydrobbiidae spp. (e.g. Caldara et al., 2008; Carboni
et al., 2010; Raynal et al., 2010). Foraminifera, diatoms and ostracod assemblages dominated dominated by
brackish littoral taxa or inner estuarine taxa (e.g Cyprideis torosa, Leptocythere lagunae, Loxoconcha elliptica,
Cytherois fischeri; e.g., Amorosi et al., 2009; Nachite et al., 2010; Marriner et al., 2012b; Marco-Barba et al.,
2013). Lower species diversity compared to the open lagoon system.
Undifferentiated brackish Marsh plant macrofossils (e.g. Silvestri et al., 2005; Serandrei-Barbero et al., 2006). Molluscs, foraminiferal, (HAT to MLW)/2 HAT to MLW
environment diatoms and ostracods assemblages dominated by freshwater-slightly brackish or swamp taxa and shallow
marine taxa (e.g. Colombaroli et al., 2007; Amorosi et al., 2013).
Beachrocks with cement Samples showing irregularly distributed needles or isopachous fibres of aragonitic cement or isopachous rims (HAT to MLW)/2 HAT to MLW
fabric or stratigraphic (bladed or fibrous) and micritic HMC cement. Small-scaled trough cross stratification or low angle seaward
information dipping tabular cross bedding with the presence of keystone vugs (e.g. Strasser et al., 1989; Vousdoukas
et al., 2007; Desruelles et al., 2009; Mauz et al., 2015b)
General beachrock Samples that do not meet the above requirements to be classified as strictly intertidal beachrocks 0.5 m MSL to 2 m
and to −1m
Limiting points
Marine limiting In situ infralittoral benthos (e.g. Sartoretto et al., 1996; Rovere et al., 2015). Identifiable marine shells in MSL Below MSL
poorly to well-bedded sandy and silty sediments typical of the upper shoreface or prodelta environments
(e.g., Sabatier et al., 2010; Marriner et al., 2012b). Posidonia oceanica beds found in open marine deposits.
Foraminiferal and ostracod assemblages dominated by marine taxa (Carboni et al., 2002; Zaîbi et al., 2011;
Amorosi et al., 2013). Lagoonal sediments that do not meet the above requirements to be classified as index
points.
Terrestrial limiting Freshwater plant macrofossils and peat with freshwater diatoms (e.g., Colombaroli et al., 2007; Di Rita et al., MSL Above MSL
2010). Upper beach deposits and terrestrial paleosoils. Foraminiferal and ostracod assemblages dominated by
freshwater taxa in swamps or fluvial environnents (Carboni et al., 2002; Milli et al., 2013; Rossi et al., 2011;
Amorosi et al., 2013).
estuary taxa (e.g., Aurila spp., Xestoleberis spp., Leptocythere spp., oxygen depletion on the lagoon bottom (Lachenal, 1989; Cimerman
Loxoconcha spp.). Finding a direct relationship between these fossil as- and Langer, 1991).
semblages and a palaeo MSL is challenging because quantitative analysis The evolution of a coastal lagoon from an open to semi-enclosed envi-
of modern analogs are seldom reported in the literature. Nonetheless, we ronment is commonly recorded in the buried lagoonal successions of the
produced index points using samples containing in situ C. glaucum mol- Mediterranean's clastic coastlines, in a so-called regressive sequence
lusc shells, a euryhaline species living in salinities of 4–100‰ and not tol- (e.g., Reineck and Singh, 1973; Caldara et al., 2008; Sabatier et al., 2010).
erant of significant aerial exposure (Nikula and Väinölä, 2003; Orrù et al., The gradual development of sandy barriers favours the progressive
2014). Since C. glaucum lives within the first 2 m of depth (Gravina et al., isolation of the brackish water body with consequent silting and evapora-
1989; Lambeck et al., 2004a; Primavera et al., 2011), we associated an in- tion (Kjerfve, 1994). This leads to significant shallowing and silting of the
dicative range from 0 to −2 MSL to the samples in outer or marine- semi-enclosed lagoons with respect to the open lagoons. The depth of
influenced lagoon facies (Fig. 3B, Table 1). these lagoons seldom exceeds a few decimetres (e.g., Ruiz et al., 2006;
ii) Inner or semi-enclosed lagoon facies show sedimentological and Vött, 2007) and the concomitant presence of macrophytes such as of
micropaleontological features typical of a brackish lagoonal/estuarine Ruppia maritima and Lamprothamnium papulosum is consistent with max-
environment with lower species diversity with respect to the open imum lagoon depths of −1 m (e.g., Primavera et al., 2011). Thus, samples
marine facies. There is a dominance of macrofossils typical of sheltered found in inner or semi-enclosed lagoon facies (usually lagoonal shells,
marine-lacustrine environments with the presence of C. glaucum, Abra plant remains, organic sediments, wood or charcoal) have an associated
segmentum, Loripes lacteus and Hydrobiidae spp. (e.g., Gravina et al., indicative range from 0 to −1m(Fig. 3B, Table 1).
1989; Sabatier et al., 2010; Marriner et al., 2012b). Foraminifera, diatom We listed as undifferentiated brackish facies (Fig. 3B, Table 1) those
and ostracod assemblages are dominated by brackish inner estuarine samples that did not provide enough data to define a clear depositional
species such as Cyprideis torosa, Leptocythere lagunae, Loxoconcha environment (e.g., Correggiari et al., 1996) or that showed the presence
elliptica,and Cytherois fischeri. The presence of opportunistic species tol- of marsh plant macrofossils together with micro- and macro-fossil
erant of restricted conditions and ample food availability, such as assemblages ranging from freshwater/slightly brackish taxa to shallow
Hyanesina germanica, Ammonia perlucida and L. elliptica, is consistent marine taxa (e.g., Colombaroli et al., 2007; Amorosi et al., 2013). For
with a low-energy, organic-rich lagoonal basin without significant these samples, we assumed an indicative range from HAT to Mean