Page 7 - Thymus_BSE
P. 7
252 L. Llorens et al. / Biochemical Systematics and Ecology 56 (2014) 246e254
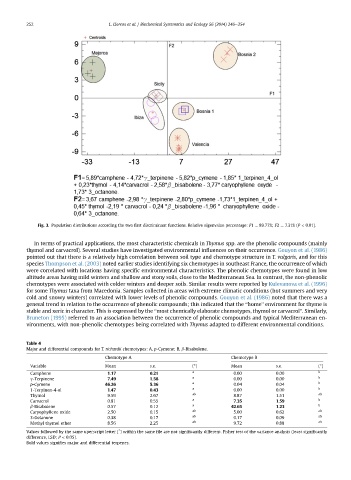
Fig. 3. Population distributions according the two first discriminant functions. Relative eigenvalue percentage: F1 ¼ 89.77%; F2 ¼ 7.31% (P < 0.01).
In terms of practical applications, the most characteristic chemicals in Thymus spp. are the phenolic compounds (mainly
thymol and carvacrol). Several studies have investigated environmental influences on their occurrence. Gouyon et al. (1986)
pointed out that there is a relatively high correlation between soil type and chemotype structure in T. vulgaris, and for this
species Thompson et al. (2003) noted earlier studies identifying six chemotypes in southeast France, the occurrence of which
were correlated with locations having specific environmental characteristics. The phenolic chemotypes were found in low
altitude areas having mild winters and shallow and stony soils, close to the Mediterranean Sea. In contrast, the non-phenolic
chemotypes were associated with colder winters and deeper soils. Similar results were reported by Kulevanova et al. (1996)
for some Thymus taxa from Macedonia. Samples collected in areas with extreme climatic conditions (hot summers and very
cold and snowy winters) correlated with lower levels of phenolic compounds. Gouyon et al. (1986) noted that there was a
general trend in relation to the occurrence of phenolic compounds; this indicated that the “home” environment for thyme is
stable and xeric in character. This is expressed by the “most chemically elaborate chemotypes, thymol or carvacrol”. Similarly,
Bruneton (1995) referred to an association between the occurrence of phenolic compounds and typical Mediterranean en-
vironments, with non-phenolic chemotypes being correlated with Thymus adapted to different environmental conditions.
Table 4
Major and differential compounds for T. richardii chemotypes: A, p-Cymene; B, b-Bisabolene.
Chemotype A Chemotype B
Variable Mean s.e. (*) Mean s.e. (*)
Camphene 1.17 0.21 a 0.00 0.00 b
g-Terpinene 7.49 1.58 a 0.00 0.00 b
p-Cymene 46.26 5.36 a 0.04 0.04 b
1-Terpinen-4-ol 1.47 0.43 a 0.00 0.00 b
Thymol 9.59 2.67 ab 8.87 1.51 ab
Carvacrol 0.81 0.55 a 7.35 1.59 b
b-Bisabolene 0.57 0.12 a 42.65 1.23 b
Caryophyllene oxide 2.50 0.15 ab 5.00 0.62 ab
3-Octanone 0.38 0.17 ab 0.17 0.09 ab
Methyl thymol ether 8.56 2.25 ab 9.72 0.88 ab
*
Values followed by the same uperscript letter ( ) within the same file are not significantly different. Fisher test of the variance analysis (least significantly
difference, LSD; P < 0.05).
Bold values signifies major and differential terpenes.