Page 8 - Caruso_Zaccone_2000
P. 8
958 G. CARUSO AND R. ZACCONE
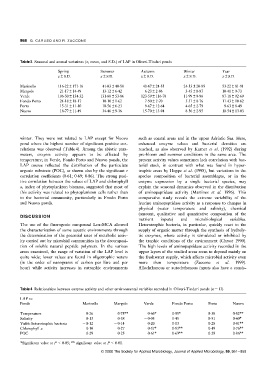
Table 3 Seasonal and annual variations (x, mean, and S.D.) of LAP in Oliveri-Tindari ponds
Spring Summer Autumn Winter Year
x S.D. x S.D. x S.D. x S.D. x S.D.
Marinello 11622 17116 4103 4058 4067 2455 2453 2085 5322 8191
Mergolo 2117 1449 1312 642 623 286 345 087 1041 973
Verde 10650 13432 13168 5386 12359 11678 1199 996 8718 9269
Fondo Porto 2418 1817 1010 162 790 270 551 076 1143 1062
Porto 1531 1110 1056 623 967 1264 465 279 963 849
Nuovo 1877 1149 3446 936 1570 1394 856 295 1854 1303
winter. They were not related to LAP except for Nuovo such as coastal areas and in the upper Adriatic Sea. Here,
pond where the highest number of signi®cant positive cor- enhanced enzyme values and bacterial densities are
relations was observed (Table 4). Among the abiotic para- reached, as also observed by Karner et al. (1992) during
meters, enzyme activity appears to be affected by pre-bloom and summer conditions in the same area. The
temperature; in Verde, Fondo Porto and Nuovo ponds, the present activity values sometimes lack correlation with bac-
LAP course re¯ected the distribution of the particulate terial stock, in contrast with what was found in hyper-
organic substrate (POC), as shown also by the signi®cant r trophic areas by Hoppe et al. (1998), but variations in the
correlation coef®cients (061; 069; 086). The strong posi- species composition of bacterial assemblages, or in the
tive correlation between the values of LAP and chlorophyll enzyme expression by a single bacterial species, may
a, index of phytoplankton biomass, suggested that most of explain the seasonal dynamics observed in the distribution
this activity was related to phytoplankton cells rather than of aminopeptidase activity (Martinez et al. 1996). This
to the bacterial community, particularly in Fondo Porto comparative study reveals the extreme variability of the
and Nuovo ponds. leucine aminopeptidase activity as a response to changes in
physical (water temperature and salinity), chemical
(amount, qualitative and quantitative composition of the
DISCUSSION
nutrient inputs) and microbiological variables.
The use of the ¯uorogenic compound Leu-MCA allowed Heterotrophic bacteria, in particular, quickly react to the
the characterization of some aquatic environments through supply of organic matter through the synthesis of hydroly-
the determination of the potential rates of metabolic activ- tic enzymes, whose activity is stimulated or inhibited by
ity carried out by microbial communities in the decomposi- the trophic conditions of the environment (Chrost 1990).
tion of soluble natural peptide polymers. In the various The high levels of aminopeptidase activity recorded in the
areas examined, the range of variation of the LAP level is upper layers of the studied areas seem to depend mainly on
quite wide; lower values are found in oligotrophic waters the freshwater supply, which affects microbial activity even
(in the order of nanograms of carbon per litre and per more than temperature (Zaccone et al. 1999).
hour) while activity increases in eutrophic environments Allochthonous or autochthonous inputs also have a consis-
Table 4 Relationships between enzyme activity and other environmental variables recorded in Oliveri-Tindari ponds (n 13)
LAP vs:
Ponds Marinello Mergolo Verde Fondo Porto Porto Nuovo
Temperature 026 075** 066* 055* 038 082**
Salinity 013 038 ÿ 005 045 051 060*
Viable heterotrophic bacteria ÿ 012 ÿ 014 020 003 028 081**
Chlorophyll a 010 027 057* 093** 049 076**
POC 029 025 061* 069** 029 086**
*Signi®cant value at P < 005; ** signi®cant value at P < 001.
= 2000 The Society for Applied Microbiology, Journal of Applied Microbiology, 89, 951ÿ959