Page 10 - Passalacqua_Peruzzi_Pellegrino2008
P. 10
Passalacqua & al. • Biosystematics of the Jacobaea maritima group TAXON 57 (3) • August 2008: 893–906
Observations on flowering period. — Floral phe- in J. ambigua. This can account for the very different
nology was observed both in the field and in cultivated taxonomic arrangements proposed by Chater & Walters
plants over a period of three years (2002–2004): the plants (1976) (see Table 1), of course unaware of molecular dif-
referable to J. maritima s.l. were in bloom from the second ferentiation. Presumably, the phenotypic convergence be-
half of May to the end of June, while the plants referable tween J. ambigua and J. bicolor can be partially explained
to J. ambigua s.l. flowered from the end of June to the end by the habitat conditions to which J. ambigua is subject,
of July or the first days of August. i.e., water and temperature stress (due to the particular
lavic substrate, together with the low altitude). Further
investigation is needed to understand the underlying
causes of the phenotypic similarity between J. ambigua
DISCUSSION and J. maritima s.l.
Molecular analysis confirms the results of Pelser Within J. ambigua s.l., J. nebrodensis and J. ambigua
& al. (2003) who found that three taxa (J. gnaphalodes showed some molecular (Fig. 3) and morphological (Fig. 4)
from Crete, J. ambigua from Greece, J. maritima from differentiation. At the molecular level, only ISSR analysis
the Western Mediterranean area) were monophyletic and showed some differentiation, while no difference was found
nested in Senecio sect. Jacobaea ; moreover, it extends this in the other analysed molecular markers. Also the morpho-
observation to other taxa of the group (all in the C. Medi- logical distinction is relatively low, and multiple charac-
terranean area), confirming a monophyletic group that ters are needed (i.e., hair covering of adaxial leaf surface;
comprises two clades (Fig. 2): J. maritima s.l. and J. am- number of septate segments; stem height and hairiness) to
bigua s.l./J. gnaphalodes. distinguish the two, since each single character is more or
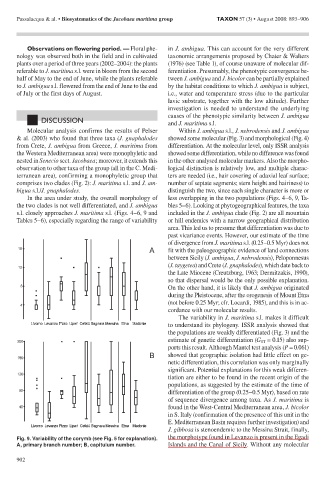
In the area under study, the overall morphology of less overlapping in the two populations (Figs. 4–6, 9, Ta-
the two clades is not well differentiated, and J. ambigua bles 5–6). Looking at phytogeographical features, the taxa
s.l. closely approaches J. maritima s.l. (Figs. 4–6, 9 and included in the J. ambigua clade (Fig. 2) are all mountain
Tables 5–6), especially regarding the range of variability or hill endemics with a narrow geographical distribution
area. This led us to presume that differentiation was due to
past vicariance events. However, our estimate of the time
of divergence from J. maritima s.l. (0.25–0.5 Myr) does not
fit with the paleogeographic evidence of land connections
between Sicily (J. ambigua, J. nebrodensis), Peloponnesus
(J. taygetea) and Crete (J. gnaphalodes), which date back to
the Late Miocene (Creutzburg, 1963; Dermitzakis, 1990),
so that dispersal would be the only possible explanation.
On the other hand, it is likely that J. ambigua originated
during the Pleistocene, after the orogenesis of Mount Etna
(not before 0.25 Myr; cfr. Locardi, 1985), and this is in ac-
cordance with our molecular results.
The variability in J. maritima s.l. makes it difficult
to understand its phylogeny. ISSR analysis showed that
the populations are weakly differentiated (Fig. 3) and the
estimate of genetic differentiation (G ST = 0.15) also sup-
ports this result. Although Mantel test analysis (P = 0.061)
showed that geographic isolation had little effect on ge-
netic differentiation, this correlation was only marginally
significant. Potential explanations for this weak differen-
tiation are either to be found in the recent origin of the
populations, as suggested by the estimate of the time of
differentiation of the group (0.25–0.5 Myr), based on rate
of sequence divergence among taxa. As J. maritima is
found in the West-Central Mediterranean area, J. bicolor
in S. Italy (confirmation of the presence of this unit in the
E. Mediterranean Basin requires further investigation) and
J. gibbosa is stenoendemic to the Messina Strait, finally,
Fig. 9. Variability of the corymb (see Fig. 5 for explanation). the morphotype found in Levanzo is present in the Egadi
A, primary branch number; B, capitulum number. Islands and the Canal of Sicily. Without any molecular
902