Page 9 - Spatial _distribution_Brugnano_2010
P. 9
Author's personal copy
C. Brugnano et al. / Journal of Marine Systems 81 (2010) 312–322 319
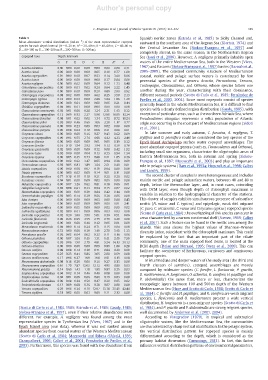
Table 3 Spanish neritic zones (Estrada et al., 1985) to Sicily Channel and,
Mean abundance vertical distribution (ind.m −3 ) of the most representative copepod eastward in the southern area of the Aegean Sea (Kiortsis, 1974) and
species for each depth interval (A=0–20 m; A*=20–40 m; B=40–60 m; C=60–80 m; the Central Levantine Sea (Siokou-Frangou et al., 1997)and
D=80–100 m; E=100–200 m; F=200–300 m; GN300 m).
completely absent, in the same season, in the Northeastern Aegean
Copepod taxa Depth intervals Sea (Isari et al., 2006). However, A. negligens primarily inhabits open
G F E D C B A* A waters of the entire Mediterranean Sea, both in the Western (Vives,
1967) and Eastern (Siokou-Frangou et al., 1997) basins (Razouls et al.,
Acartia adriatica 0.00 0.00 0.00 0.00 0.00 0.00 0.09 0.71
Acartia clausi 0.00 0.00 0.00 0.00 0.05 0.10 0.77 1.09 2005–2009). The copepod community structure of Mediterranean
Acartia copepodites 0.01 0.00 0.00 0.07 0.53 0.14 3.60 8.66 coastal, neritic and pelagic surface waters is constituted by few
Acartia danae 0.00 0.00 0.00 0.00 0.00 0.17 0.04 0.00 perennial species of the genera Acartia, Paracalanus, Temora,
Acartia negligens 0.00 0.00 0.02 0.00 0.64 0.12 1.73 6.48
Calocalanus copepodites 0.00 0.00 0.01 0.02 0.26 0.64 2.32 1.45 Centropages, Clausocalanus, and Oithona, whose species follow one
Calocalanus pavo 0.00 0.00 0.00 0.00 0.20 0.89 2.00 0.92 another during the year, characterizing with their dominance,
Centropages copepodites 0.08 0.02 0.00 0.00 6.62 8.25 3.50 2.13 different seasonal periods (Scotto di Carlo et al., 1985; Fernàndez de
Centropages typicus 0.16 0.00 0.03 0.00 2.68 3.64 1.86 1.29 Puelles et al., 2003, 2004). Since most copepods consist of species
Centropages violaceus 0.00 0.00 0.01 0.00 0.00 0.05 0.21 0.44 generally found in the whole Mediterranean Sea, it is difficult to find
Chiridius copepodites 0.04 0.01 0.01 0.00 0.00 0.00 0.00 0.00
Clausocalanus arcuicornis 0.02 0.01 0.08 0.28 1.24 2.31 1.43 0.12 species with a clearly defined region distribution (Gaudy, 1985), with
Clausocalanus copepodites 0.12 0.09 0.53 2.27 5.98 12.08 18.05 13.24 exception of particular areas, such as the northern Adriatic Sea, where
Clausocalanus furcatus 0.08 0.01 0.02 0.02 1.34 1.72 8.72 10.24 Pseudocalanus elongatus represents a relict population of Atlantic
Clausocalanus jobei 0.02 0.00 0.46 0.65 1.06 2.38 1.17 0.77 origin not occurring in the most part of Mediterranean regions (Sidoti
Clausocalanus paululus 0.00 0.00 0.23 0.09 0.03 0.02 0.00 0.00 et al., 2001).
Clausocalanus pergens 0.00 0.08 0.04 0.10 0.06 0.31 0.08 0.01
Corycaeus clausi 0.00 0.00 0.00 0.16 0.27 0.43 0.62 0.09 In late summer and early autumn, C. furcatus, A. negligens, T.
Corycaeus copepodites 0.06 0.03 0.55 2.74 4.66 4.42 4.23 2.40 stylifera and O. plumifera could be considered the key species of the
Corycaeus flaccus 0.00 0.00 0.00 0.00 0.01 0.05 0.19 0.12 Egadi Island Archipelago surface water copepod assemblages. The
Corycaeus furcifer 0.16 0.19 1.54 3.92 1.94 1.13 0.39 0.70 most abundant copepod genera (such as, Clausocalanus and Oithona),
Corycaeus giesbrechti 0.02 0.00 0.00 0.00 0.32 0.08 0.42 1.92
Corycaeus latus 0.02 0.00 0.00 0.04 0.13 0.35 0.94 1.54 including small size organisms, characterize the oligotrophic areas of
Corycaeus typicus 0.06 0.05 0.35 0.73 0.68 1.21 1.05 0.36 Eastern Mediterranean Sea, both in autumn and spring (Siokou-
Ctenocalanus copepodites 0.00 0.02 0.62 3.47 8.65 8.94 0.38 0.09 Frangou et al., 1997; Mazzocchi et al., 2003) and play an important
Ctenocalanus vanus 0.02 0.05 0.83 1.89 3.54 2.51 0.40 0.01 role in pelagic systems (Dam et al., 1993a; Hopcroft et al., 1998; Calbet
Diaixis copepodites 0.00 0.00 0.06 0.07 0.19 0.04 0.00 0.00
Diaixis pigmoea 0.00 0.00 0.02 0.09 0.14 0.01 0.11 0.00 and Landry, 1999).
Eucalanus copepodites 0.77 0.18 0.10 0.18 0.21 0.22 0.26 0.02 The second cluster of samples is more heterogeneous and includes
Eucalanus crassus 0.04 0.01 0.00 0.00 0.02 0.05 0.00 0.00 mainly neritic and pelagic subsurface waters, between 40 and 80 m
Haloptilus copepodites 0.00 0.07 1.26 0.62 0.81 0.18 0.06 0.01 depth, below the thermocline layer, and, in most cases, coinciding
Haloptilus longicornis 0.02 0.08 0.61 0.33 0.54 0.15 0.07 0.02 with DCM layer, even though depth of chlorophyll maximum is
Heterorhabdus copepodites 0.00 0.01 0.09 0.39 0.84 0.42 0.14 0.00
Heterorhabdus papilliger 0.02 0.03 0.14 0.30 0.56 0.38 0.06 0.00 variable in relation to the hydrographical character of the stations.
Isias clavipes 0.00 0.00 0.00 0.00 0.03 0.00 0.00 0.43 This cluster of samples exhibits simultaneous presence of subsurface
Isias copepodites 0.00 0.00 0.00 0.00 0.09 0.00 0.01 2.41 neritic (N. minor and C. typicus) and epipelagic, weak diel migrant
Lubbockia copepodites 0.00 0.00 0.05 0.00 0.00 0.02 0.09 0.02 (C. pavo, C. arcuicornis, C. vanus and Corycaeus typicus) copepod species
Lubbockia squillimana 0.02 0.00 0.01 0.00 0.06 0.03 0.03 0.00
Lucicutia copepodites 0.02 0.20 1.06 3.91 5.01 1.36 0.51 0.06 (Scotto di Carlo et al., 1984); the overlapping of this species can occur in
Lucicutia flavicornis 0.06 0.26 0.95 3.55 2.75 0.79 0.29 0.00 areas characterized by a narrow continental shelf (Soenen, 1969; Calbet
Lucicutia longicornis 0.00 0.01 0.08 0.48 0.12 0.05 0.01 0.00 et al., 2001). Such a feature can be found in the neritic area among the
Mesocalanus tenuicornis 0.04 0.00 0.14 0.24 0.71 0.15 0.04 0.00 islands. This area shows the highest values of Shannon–Wiener
Nannocalanus minor 0.33 0.00 0.08 0.19 1.49 2.70 3.45 1.13 diversity index, coincident with the chlorophyll maximum. This could
Neocalanus gracilis 0.02 0.03 0.04 0.02 0.33 0.11 0.16 0.06
Oithona plumifera 0.00 0.07 1.06 0.45 1.31 1.41 2.49 7.59 be explained by the fact that an important part of the ciliate
Oithona copepodites 0.02 0.06 1.90 2.79 4.81 5.24 14.13 30.13 community, one of the main copepod food items, is located at the
Oithona atlantica 0.00 0.00 0.00 0.00 0.09 0.89 1.80 0.28 DCM depth (Dolan and Marrasè, 1995; Perez et al., 2000). This can
Oncaea conifera 0.08 0.08 0.14 0.39 0.71 0.84 0.20 0.00 facilitate the coexistence of herbivorous, carnivores and omnivorous
Oncaea copepodites 0.25 0.23 0.41 0.86 3.05 2.00 0.59 0.08 copepod species.
Oncaea mediterranea 0.17 0.06 0.37 1.09 1.08 0.61 0.15 0.08
Pleuromamma abdominalis 0.08 0.18 0.20 0.01 0.16 0.27 0.13 0.00 In intermediate and deeper waters of the study area (the third and
Pleuromamma copepodites 0.61 1.79 7.87 12.03 12.12 4.95 0.80 0.03 fourth clusters of samples), copepod assemblages are mostly
Pleuromamma gracilis 0.14 0.60 1.43 1.18 1.99 0.82 0.35 0.03 composed by midwater species (C. furcifer, L. flavicornis, P. gracilis,
Scaphocalanus copepodites 0.04 0.02 0.14 0.46 0.40 0.08 0.00 0.00 O. mediterranea, H. longicornis, O. atlantica, O. conifera, H. papilliger and
Scaphocalanus curtus 0.04 0.01 0.09 0.66 0.60 0.19 0.03 0.00
Scolecithricella copepodites 0.06 0.08 0.24 0.51 0.47 0.20 0.05 0.02 P. abdominalis) the same that, more or less, characterizes the
Scolecithricella dentata 0.12 0.09 0.08 0.26 0.38 0.07 0.00 0.00 mesopelagic layers between 100 and 500 m depth of the Western
Temora copepodites 0.29 0.06 0.34 0.11 12.07 13.18 35.45 42.44 Mediterranean Sea (Hure and Scotto di Carlo, 1968; Scotto di Carlo et
Temora stylifera 0.18 0.02 0.05 0.03 0.60 1.11 2.12 6.00 al., 1984). C. furcifer and H. papilliger, and O. conifera are weak migrant
species, L. flavicornis and O. mediterranea present a wide vertical
distribution, H. longicornis is a non-migrant species (Scotto di Carlo et
(Scotto di Carlo et al., 1984, 1985; Estrada et al., 1985; Gaudy, 1985; al., 1984), and P. gracilis and P. abdominalis are strong migrant species,
Siokou-Frangou et al., 1997), even if their relative abundances were well documented by Andersen et al. (2001, 2004).
different. For example, A. negligens was found among the most According to Vinogradov (1970), in tropical and subtropical
representative species in Tyrrhenian Sea (Vives, 1967) and in the oligotrophic waters, like the Mediterranean Sea, the communities
Egadi Island area (our data), whereas it was not ranked among are characterized by sharp vertical stratification. In the pelagic system,
abundant species from coastal waters of the Western Mediterranean the vertical distribution pattern for copepod species is mainly
(Scotto di Carlo et al., 1984; Mazzocchi and Ribera d'Alcalà, 1995; differentiated according to the depth, which is considered as the
Champalbert, 1996; Calbet et al., 2001; Fernàndez de Puelles et al., primary habitat dimension (Cummings, 1983). In fact, this factor
2003). Furthermore, this species was found with low abundance from influences vertical distribution patterns of environmental parameters,