Page 8 - Spatial _distribution_Brugnano_2010
P. 8
Author's personal copy
318 C. Brugnano et al. / Journal of Marine Systems 81 (2010) 312–322
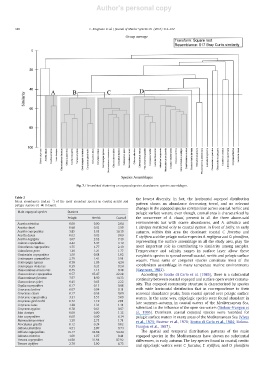
Fig. 7. Hierarchical clustering on copepod species abundances: species assemblages.
Table 2 the lowest diversity. In fact, the horizontal copepod distribution
Mean abundances (ind.m −3 ) of the most abundant species in coastal, neritic and
pelagic stations (0–40 m layer). pattern shows an abundance decreasing trend, and no relevant
changes in the copepod species composition across coastal, neritic and
Main copepod species Stations pelagic surface waters, even though, coastal area is characterized by
Pelagic Neritic Coastal the occurrence of A. clausi, present in all the three above-said
environments but with scarce abundances, and A. adriatica and
Acartia adriatica 0.00 0.00 2.04
Acartia clausi 0.68 0.02 3.59 I. clavipes restricted only to coastal system in front of Sicily. In early
Acartia copepodites 3.85 3.65 16.10 autumn, within this area the dominant coastal C. furcatus and
Acartia danae 0.03 0.02 0.00 T. stylifera and the pelagic surface species A. negligens and O. plumifera,
Acartia negligens 4.67 2.92 7.49
Calanus copepodites 4.42 5.37 3.10 representing the surface assemblage in all the study area, play the
Calocalanus copepodites 1.75 1.77 2.10 most important role in contributing to similarity among samples.
Calocalanus pavo 1.36 1.21 1.77 Temperature and salinity ranges in surface layer allow these
Candacidae copepodites 1.50 0.68 1.92 eurybiotic species to spread overall coastal, neritic and pelagic surface
Centropages copepodites 2.79 1.41 5.91
Centropages typicus 0.38 1.09 4.24 waters. These same or congener species constitute most of the
Centropages violaceus 0.39 0.33 0.24 zooplankton assemblage in many temperate marine environments
Clausocalanus arcuicornis 0.25 1.13 0.28 (Raymont, 1983).
Clausocalanus copepodites 9.57 15.47 22.28 According to Scotto di Carlo et al. (1985), there is a substantial
Clausocalanus furcatus 7.57 8.50 14.23 continuity between coastal copepod and surface open water commu-
Clausocalanus jobei 0.45 0.65 2.22
Copilia copepodites 0.17 0.12 0.68 nity. This copepod community structure is characterized by species
Corycaeus brehmi 0.07 0.04 0.11 with wide horizontal distribution that in correspondence to their
Corycaeus clausi 0.17 0.55 0.00 seasonal abundance peaks, from coastal spread over pelagic surface
Corycaeus copepodites 3.31 3.51 3.09 waters. In the same way, epipelagic species were found abundant in
Corycaeus giesbrechti 0.72 1.19 2.01
Corycaeus latus 1.28 1.31 1.11 late summer–autumn, in coastal waters of the Mediterranean Sea,
Corycaeus typicus 0.78 0.61 0.67 submitted to the influence of the open sea waters (Siokou-Frangou et
Isias clavipes 0.00 0.00 1.13 al., 1995). Dominant coastal copepod species were recorded for
Isias copepodites 0.00 0.00 6.34 pelagic surface waters in many areas of the Mediterranean Sea (Vives
Nannocalanus minor 1.30 2.73 1.71 et al., 1975; Pasteur et al., 1976; Scotto di Carlo et al., 1984; Siokou-
Neocalanus gracilis 0.12 0.24 0.02
Oithona plumifera 6.51 2.87 9.74 Frangou et al., 1997).
Oithona copepodites 16.17 13.86 54.44 The spatial and temporal distribution patterns of the main
Oithona atlantica 1.09 1.07 0.41 copepod species in the Mediterranean have shown no substantial
Temora copepodites 14.50 31.94 87.92 differences, in early autumn. The key species found in coastal, neritic
Temora stylifera 2.70 3.90 6.73
and epipelagic waters were C. furcatus, T. stylifera, and O. plumifera